Clinical Research Solutions and Services
Biostatistics plays a very crucial role in clinical trials, regulatory submissions, and marketing efforts. Our biostatisticians utilise their extensive expertise to guide you through the complexities of study design, sample size, number of studies, analysis methods, data presentations, and interpretations. Our team of highly trained biostatisticians and SAS programmers are committed to providing high-quality services.
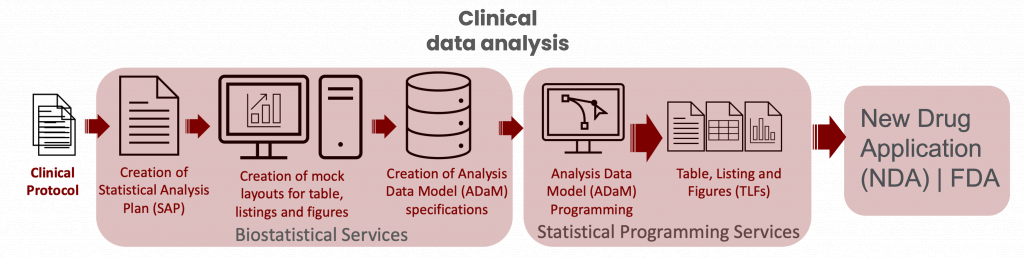
Biostatistics services
Our professional biostatisticians specialise in designing and planning clinical trials. They work closely with the study team to ensure that the trial is properly designed to answer the research question and to meet regulatory requirements.
Our biostatisticians use their extensive knowledge and experience to help determine the appropriate study design, sample size, randomization, and statistical analysis methods for the trial. They also provide input on data collection, data monitoring, and data analysis plans.
In addition, we ensure that the study is properly powered to detect differences between treatments or interventions, and for conducting simulations to assess the robustness of the study design.
Our expertise in study design, statistical analysis, and regulatory requirements helps to ensure that the trial is properly designed and that the results are accurate and reliable.
Biostatistics play a very crucial role in PK/PD (pharmacokinetic/pharmacodynamic) analysis and modeling. Biostatisticians use statistical and mathematical expertise to help interpret the data generated by PK/PD studies and to develop models that describe the relationship between the dose of a drug and the response in the body.
Our biostatisticians collaborate with the pharmacologists and other members of the study team to design PK/PD studies, analyze the data, and develop mathematical models that can be used to predict the behaviour of the drug in the body. They also use their expertise to ensure that the models are properly calibrated and validated.
Our biostatisticians use a range of statistical techniques to analyze PK/PD data, such as non-compartmental analysis, compartmental analysis, and population pharmacokinetic modeling. In certain cases we also implement more advanced techniques, such as physiologically-based pharmacokinetic modeling (PBPK) and systems pharmacology modeling.
The results of PK/PD analysis and modeling are critical to drug development, as they provide insight into the pharmacokinetic and pharmacodynamic properties of a drug, such as its absorption, distribution, metabolism, and elimination. This information can be used to optimize the dosing regimen of the drug and to predict its safety and efficacy in different patient populations.
Biostatisticians play an important role in interim analysis in clinical trials. Interim analysis involves reviewing the accumulating data from an ongoing clinical trial at predetermined intervals to assess the effectiveness and safety of the intervention being studied. This helps to ensure that the trial is on track, and can also help to identify potential safety issues or other concerns that might require modification of the study protocol.
During interim analysis, our biostatisticians use their statistical expertise to assess the accumulating data, to monitor the progress of the study, and to provide feedback to the study team. They help to ensure that the interim analysis is conducted in a rigorous and unbiased manner, and that any conclusions drawn from the data are reliable.
Our biostatisticians also play a role in determining the timing and frequency of interim analyses, as well as in deciding when to stop a trial early if the intervention is shown to be particularly effective or ineffective.
In addition, our biostatisticians could also work with the study team to adjust the sample size of the trial based on the interim results. This can help to ensure that the study is adequately powered to detect differences between treatment groups and can also help to minimize the overall cost and duration of the trial.
Biostatisticians play a crucial role in survival analysis, which is a statistical method used to analyze the time to an event of interest, such as time to death, time to disease progression, or time to relapse. Survival analysis is commonly used in clinical trials and other medical studies, where it is important to understand the time to an event of interest and to evaluate the effectiveness of interventions on this outcome.
In survival analysis, our biostatisticians use their statistical expertise to design the study and to analyze the data generated from the study. They sometimes work with the study team to determine the appropriate sample size and power for the study, and to select appropriate statistical methods for the analysis.
Biostatisticians also play a critical role in determining the appropriate endpoints for the study, such as overall survival or progression-free survival. They help to ensure that the endpoints are clinically meaningful and that the study is adequately powered to detect differences between treatment groups.
During the analysis of survival data, biostatisticians use a variety of statistical methods, such as the Kaplan-Meier method, Cox proportional hazards model, and parametric survival models. They also help to interpret the results of the analysis and to present the findings in a clear and concise manner.
Adaptive clinical trials are studies that allow for modifications to the study design during the trial based on accumulated data. Our biostatisticians play a critical role in the design and analysis of adaptive clinical trials.
During the planning phase of an adaptive clinical trial, our biostatisticians work with the study team to design the trial with the necessary flexibility to allow for adaptation. They help to select the appropriate statistical methods and to determine the stopping rules for the trial.
During the trial, our biostatisticians monitor the accumulating data and evaluate the trial’s progress. They use their statistical expertise to determine whether changes to the study design are necessary, such as changes to the sample size, the randomization scheme, or the treatment allocation. They may also help to identify potential sources of bias or confounding and recommend appropriate adjustments to the study design.
At the end of the trial, biostatisticians analyze the data and help to interpret the results. They may also help to develop new statistical methods to analyze the data from adaptive clinical trials.
Biostatisticians play a critical role in the design and analysis of bioequivalence and biosimilar trials. These trials are designed to compare the pharmacokinetics (PK) and/or pharmacodynamics (PD) of a test drug to that of a reference drug.
During the planning phase, our biostatisticians work with the study team to design the trial, including determining the appropriate sample size and statistical analysis methods. They help to select the appropriate endpoints, such as maximum plasma concentration (Cmax) and area under the curve (AUC), and determine the bioequivalence or biosimilarity criteria. They may also help to determine the appropriate statistical test, such as a two one-sided test (TOST) or an equivalence test.
During the trial, biostatisticians monitor the data and perform statistical analyses to compare the PK and/or PD of the test drug to that of the reference drug. They may also perform sensitivity analyses to evaluate the robustness of the results to different assumptions or statistical methods.
At the end of the trial, biostatisticians analyze the data and help to interpret the results. They may also help to develop new statistical methods to analyze the data from bioequivalence and biosimilar trials.
Biostatisticians play a critical role in the validation of biomarkers, which are used to indicate disease status or predict response to treatment.
During the planning phase, biostatisticians work with the study team to design the validation study, including determining the appropriate sample size and statistical analysis methods. They help to select the appropriate endpoints, such as sensitivity, specificity, positive predictive value, and negative predictive value, and determine the validation criteria. They may also help to determine the appropriate statistical test, such as a receiver operating characteristic (ROC) curve analysis.
During the validation study, biostatisticians monitor the data and perform statistical analyses to evaluate the performance of the biomarker. They may also perform sensitivity analyses to evaluate the robustness of the results to different assumptions or statistical methods.
At the end of the study, biostatisticians analyze the data and help to interpret the results. They may also help to develop new statistical methods to validate biomarkers and assess their clinical utility.
Biostatisticians play an important role in SDTM (Study Data Tabulation Model) conversion programming and validation.
SDTM is a standard format for the submission of clinical trial data to regulatory authorities, and biostatisticians help to ensure that the data are properly formatted and that the conversion process is accurate and reliable.
During the SDTM conversion process, biostatisticians may work with the clinical data management team to review the data and ensure that it is complete and accurate. They may also work with the SAS programmers to develop the conversion programs and validate the output to ensure that it meets the requirements of the standard.
Biostatisticians also play a role in the validation of the converted data. They may perform statistical analyses on the data to ensure that it is consistent with the original data and that there are no errors in the conversion process. They may also work with the clinical team to interpret the data and ensure that it meets the requirements of the regulatory authorities.
SAS Programming services
Our SAS (Statistical Analysis System) programmers help in generating Tables, Figures, and Listings (TFLs) for clinical trial analysis.
TFLs are a critical component of the regulatory submission process for clinical trials, and they provide a summary of the study data that is used to support the safety and efficacy of the investigational product. The SAS programmers work closely with the biostatisticians and the clinical team to understand the requirements for the TFLs.
Our SAS programmers ensure the accuracy and completeness of the TFLs. They may develop SAS macros and programs to automate the process and ensure that the TFLs are generated consistently across multiple studies. They also perform quality checks on the TFLs to ensure that they are accurate and consistent with the study data.
In addition, our SAS programmers provide support for the interpretation of the TFLs. They may work with the biostatisticians and the clinical team to provide additional analyses or data summaries to support the regulatory submission.
Patient profiles are used to provide a detailed summary of the individual patient data in a clinical trial. They typically include demographic information, medical history, study treatment information, and clinical outcomes. Customized patient profiles are tailored to specific study objectives or stakeholder needs and can provide a detailed overview of patient characteristics and treatment outcomes.
Our SAS programmers use their expertise in data manipulation and programming to extract the necessary patient data from the clinical trial database. We use SAS software to clean and transform the data, and to generate the patient profile outputs in a customized format that meets the specific study objectives or stakeholder needs.
Our SAS programmers develop SAS macros or programs to automate the process and ensure consistency across multiple studies. We also use SAS graphics and visualization tools to provide graphical summaries of patient data.
In addition, our SAS programmers work closely with the clinical team to ensure the accuracy and completeness of the patient profiles. We provide support for data interpretation and additional analyses to support the study objectives.
SAS programming is an essential component of clinical data management. Clinical data managers work closely with SAS programmers to ensure the accuracy, completeness, and consistency of clinical trial data.
SAS programmers provide programming support for clinical data management activities such as data cleaning, data transformation, and data validation. We use SAS software to manipulate, transform, and validate the clinical trial data to ensure that it is of high quality and meets regulatory requirements.
Our SAS programmers also play a critical role in the development of clinical data listings, which are used to display summary data from the clinical trial. These listings can include tables, graphs, and figures that summarize the clinical data and can help the clinical team to identify potential safety issues, evaluate the efficacy of the drug, and make informed decisions about the trial.
Our SAS programmers work closely with clinical data managers to ensure that the clinical data listings are accurate and consistent with the clinical trial data. We develop SAS macros or programs to automate the process of generating the data listings and to ensure consistency across multiple studies.
SAS programming plays a critical role in the generation of Annual Safety Reports (ASRs). The ASR is a comprehensive report that summarizes the safety profile of a drug or device over the course of a year. The ASR includes information on adverse events, serious adverse events, and other safety-related data.
Our SAS programmers assist in the development of ASRs by providing programming support for the analysis and reporting of safety data. They develop SAS programs to perform data checks, data cleaning, and data validation to ensure the accuracy and completeness of the safety data.
Our SAS programmers play a critical role in the generation of pooled datasets and tables, figures, and listings (TFLs) for integrated summary of safety (ISS) and integrated summary of efficacy (ISE) analyses.
Pooled datasets are typically created by combining individual study datasets from multiple clinical trials. Our SAS programmers perform data transformations, including merging and appending of datasets, to create the pooled dataset. They also perform data cleaning and validation to ensure the accuracy and completeness of the pooled dataset.
Once the pooled dataset is created, SAS programmers generate the necessary TFLs for the ISS and ISE. These TFLs are used to summarize the safety and efficacy data from all the individual studies included in the analysis.
The ISS TFLs typically include tables and figures summarizing the adverse events, serious adverse events, deaths, and other safety data across all studies. The ISE TFLs typically include tables and figures summarizing the efficacy data across all studies, including patient demographics, efficacy endpoints, and treatment effects.
What our customers like about us?
๏Time-efficient: We consistently complete and deliver projects within the agreed timeline.
๏High-Quality: Reliable and flawless results, repeatedly confirmed by hundred percent independent programming.
๏Excellent cooperation: You get easy and direct access to our dedicated biostatisticians and programmers for any task.
๏High economic efficiency: Experience the effectiveness of our standardised workflows, skilful international site selection and experienced programmers.
๏ Independent programming: We follow independent programming model with 100% Quality Control (QC) with statistical reviews
